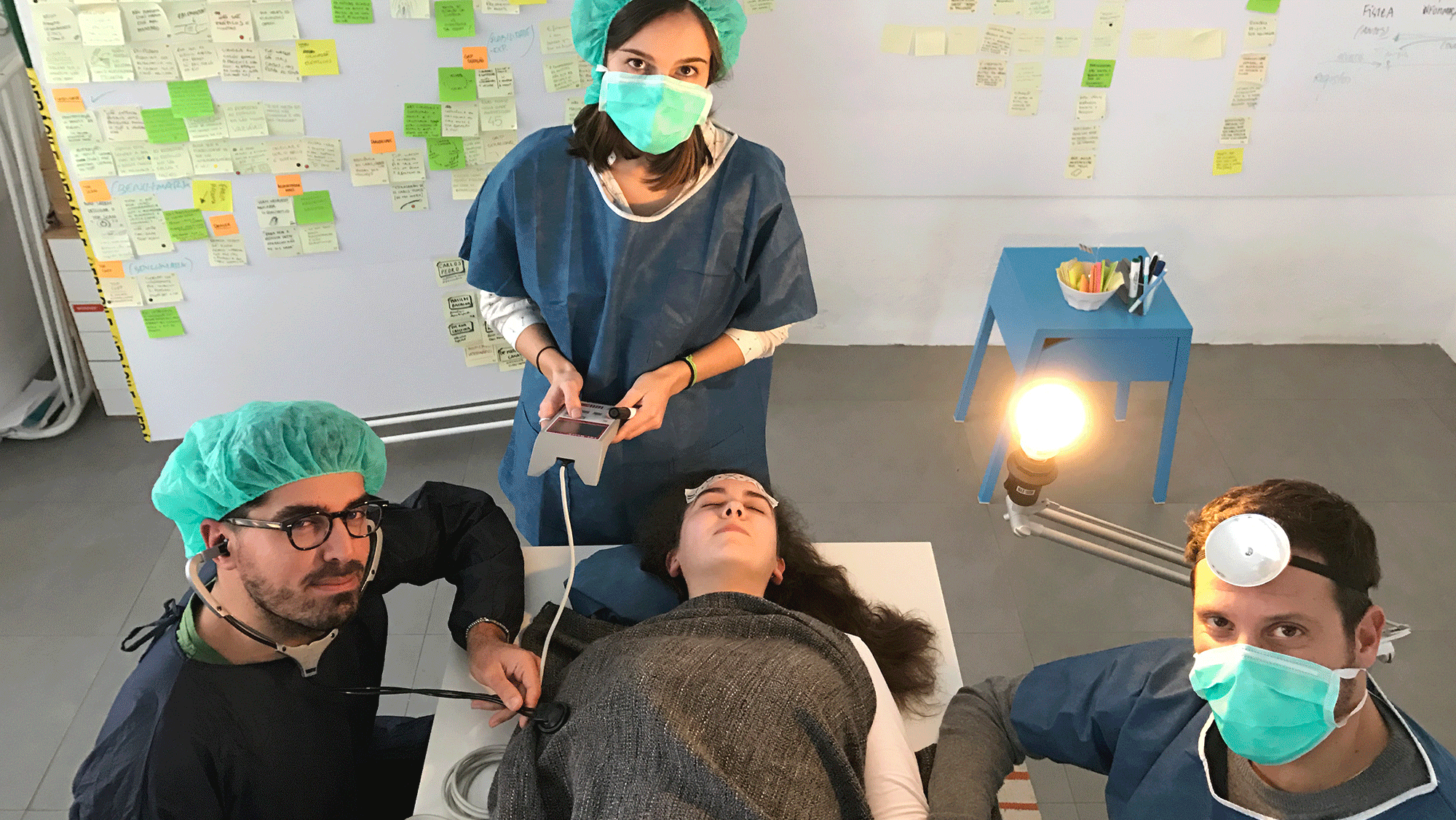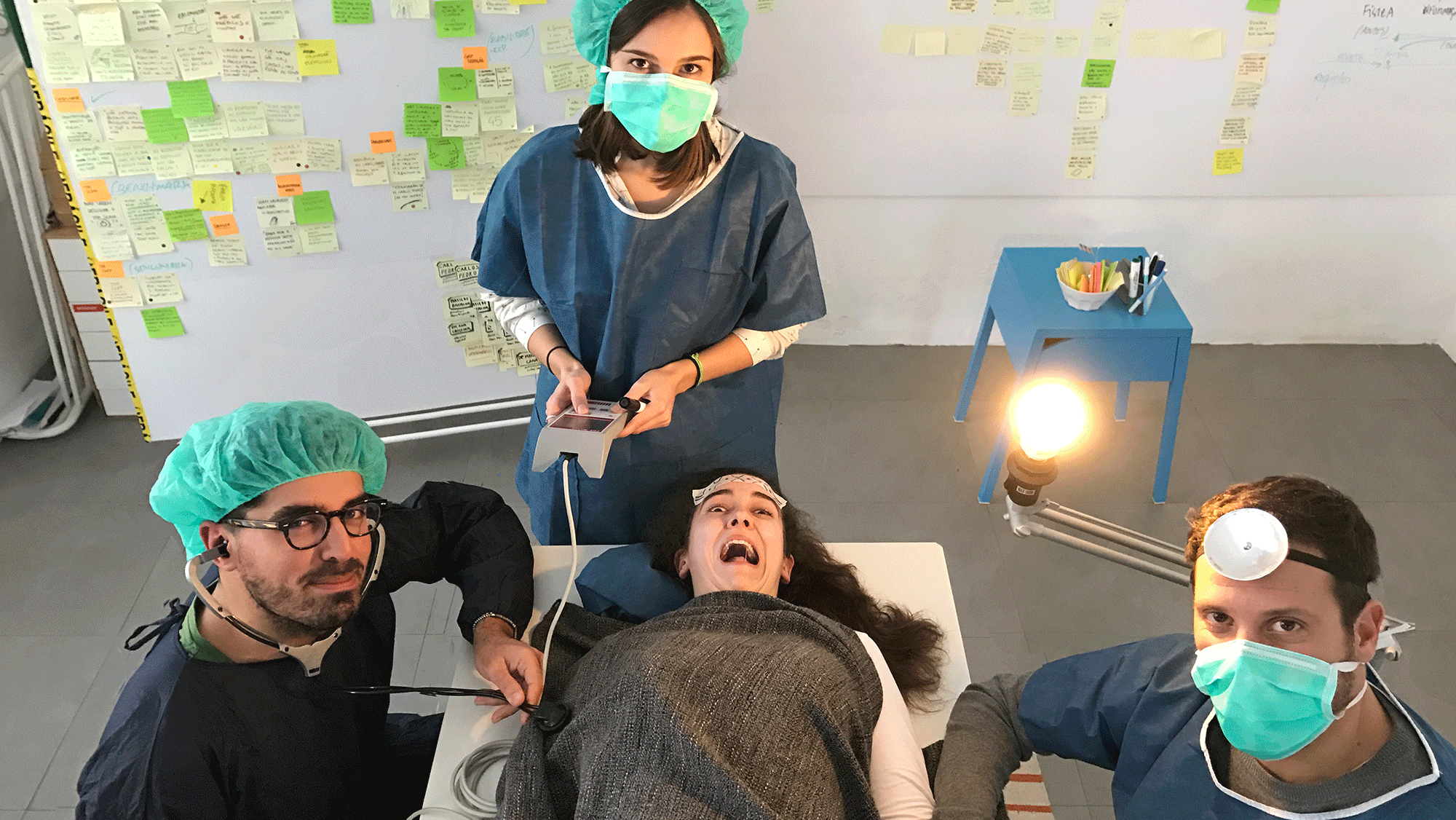
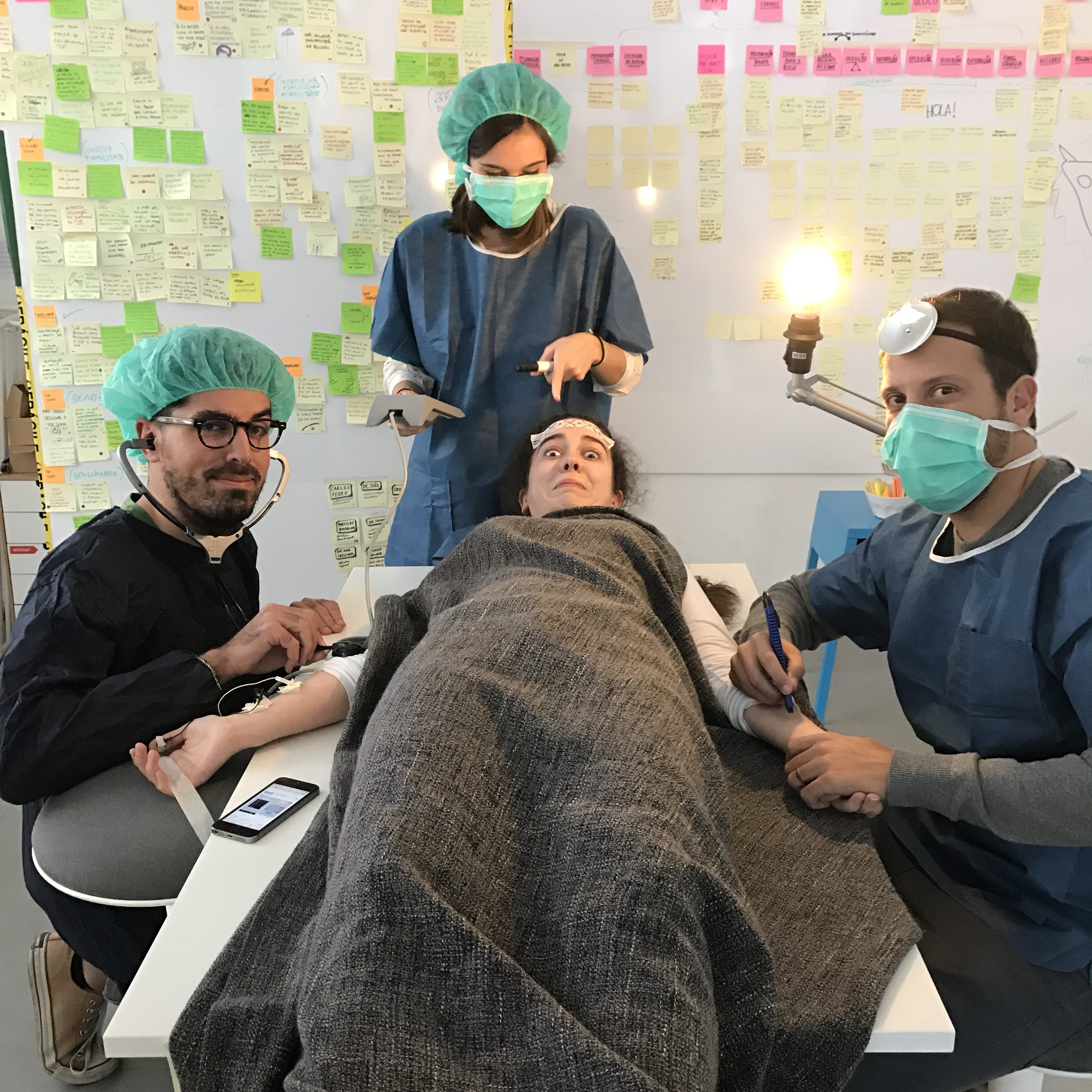
Going deep into anaesthesiology.
Product Validation
With MSD Merck Sharp & Dohme
Challenge
To rethink a medical monitoring device to monitor neuromuscular blockade, for and with anaesthesiologists.
Outcome
A validated prototype of the ideal device, with recommendations on the type of material, characteristics, and connectivity.
- Client
- MSD Merck Sharp & Dohme
- Service
- Product Validation
- Location
- Portugal
- Duration
- 8 weeks
- fields
- Strategy, Research, Prototyping, Validation, Business Case
- team
- Tiago Nunes,
João Joaquim
,Matilde Horta
,Inês Martins

CONTEXT
Neuromuscular blocking agents are usually administered during anaesthesia to facilitate endotracheal intubation, optimise surgical conditions, and assist with mechanical ventilation. Very few anaesthesiologists make use of the devices that allow them to monitor neuromuscular blockade. We dived deep into the world of anaesthesiology to understand why. Were there enough devices available? Why were the available ones not being used? How could we design a device that did meet the needs of anaesthesiologists?
Portuguese anaesthesiologists were trying to change the future of this medical branch, and while there were many ideas, there weren’t many certainties. It all changed when designers and anaesthesiologists came together.
OUR ROLE
01
Understand the problems behind the original device.
02
Redesign the medical device and validate it among anaesthesiologists.
RESEARCH
From field observations to interviews with anaesthesiologists, we were lucky enough to go behind the scenes to find out a bit more about this speciality’s crucial role in an operating room.
After putting on some scrubs, we walked along operating room halls and observed surgeries. It helped to understand how important it is for the anaesthesiologist to be in control, and how that had to be taken into account in the solution we were going to design.
We interviewed different stakeholders, but we mainly talked with anaesthesiologists. They were the end-users of the device we were designing after all. Their input was crucial in creating just the right prototype.

68
Survey respondents
21
Interviews
7
Hospitals visited
5
Hours
spent in operating rooms
1
Hypnosis session
to simulate ‘going under’
FINDINGS
Doctor doctor, give me the (bad) news.
After talking to a large number of anaesthesiologists, we were left with a number of findings. We discovered that these devices:
- were not prepared for constant and intensive maintenance and were hard to work with
- were scarcely available in hospitals and, at times, weren’t even functioning
- represented a generational gap, meaning that older anaesthesiologists weren’t as prone to use the device, and didn’t see much value in it
We also found that the original device required anaesthesiologists to have a higher mental and physical role than necessary during surgeries which, in turn, led many to perceive it as an unnecessary extra step.
Incorrect readings + difficulty when placing its electrodes + habit of relying on clinical evaluations = Unreliable device

Doctor doctor, give me the (good) news.
We did, however, also find that this device allows anaesthesiologists to:
- better anticipate complications
- keep control of the situation with more informed decisions
- benefit from greater security in case of liability
In order for this device to become more relevant, we knew its characteristics must approach those of the mandatory monitoring — from the ECG to the pulse oximeter — which doesn’t require as much of a physical and mental role from anaesthesiologists. Above all, we knew it couldn’t be seen as just one more device.
We couldn’t help but wonder: could improving the usability of this medical device revolutionise the operating room?
Prototyping
Above all, we confirmed the need to develop a solution that didn’t disrupt the environment it would exist in but that, instead, seamlessly blended itself in it. We knew what we were aiming for:
- a simple, affordable, and efficient device
- easily cleanable and adjustable
- takes up as little space as possible
- can be accessed remotely
For anaesthesiologists, the solution had to promote autonomy, allow anticipation, facilitate interpretation, increase access to knowledge, and guarantee control. To become indispensable, the solution had to be reliable, affordable, easy to use, robust, and contextual.



Research results aren’t usually presented to the people we interview. However, since we wanted to come up with a solution that was truly useful for anaesthesiologists, we needed to have their contribution. At the end of our co-creation session, different groups of anaesthesiologists whom we had previously talked to prototyped new solutions from the research findings we presented.
We had a fresh new prototype that we tested at the end of each round of interviews; it not only gave us continuous feedback, it also engaged the people we talked to, and it showed them how a concept could be translated into something real.
We took the ideas and prototypes from the co-creation session and merged them into one single solution. We validated it with the people who mattered the most, and then went back to the field to test our prototype in different hospitals. After 33 tests, we only needed a few adjustments.

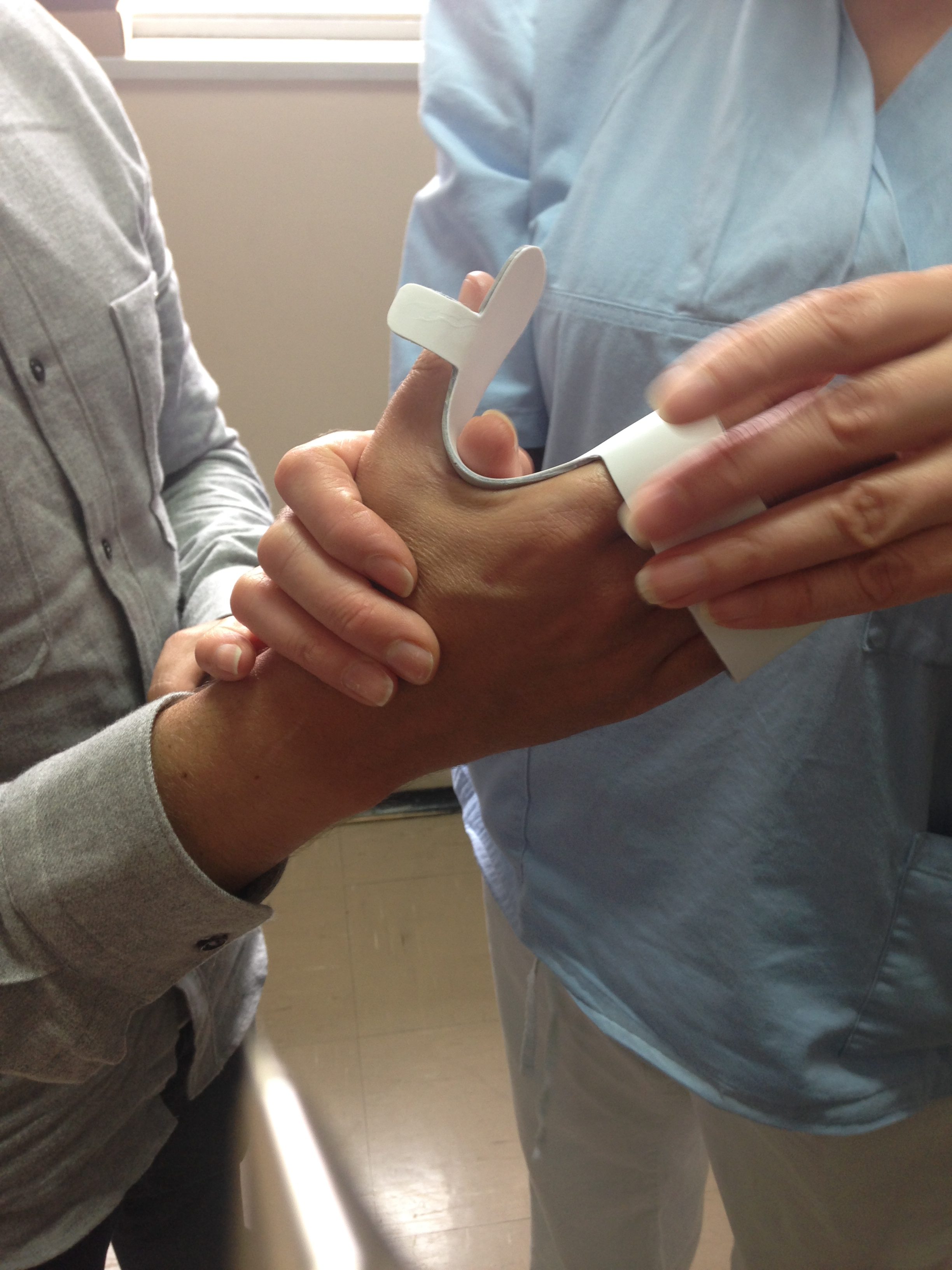
REsults
Our final ‘prescription’ was made up of three components.
- a portable stimulator
- electrodes
- a tablet interface
After another round of interviews with anaesthesiologists, we reached a consensus as to what would be the ideal solution:
- an intuitive interface
- projected onto an additional monitor in the operating room
- wirelessly connected to a pair of portable electrodes
- adjustable for different types of hands
- rigid enough to immobilise fingers
€300
How much a third of anaesthesiologists would pay to buy this device themselves.
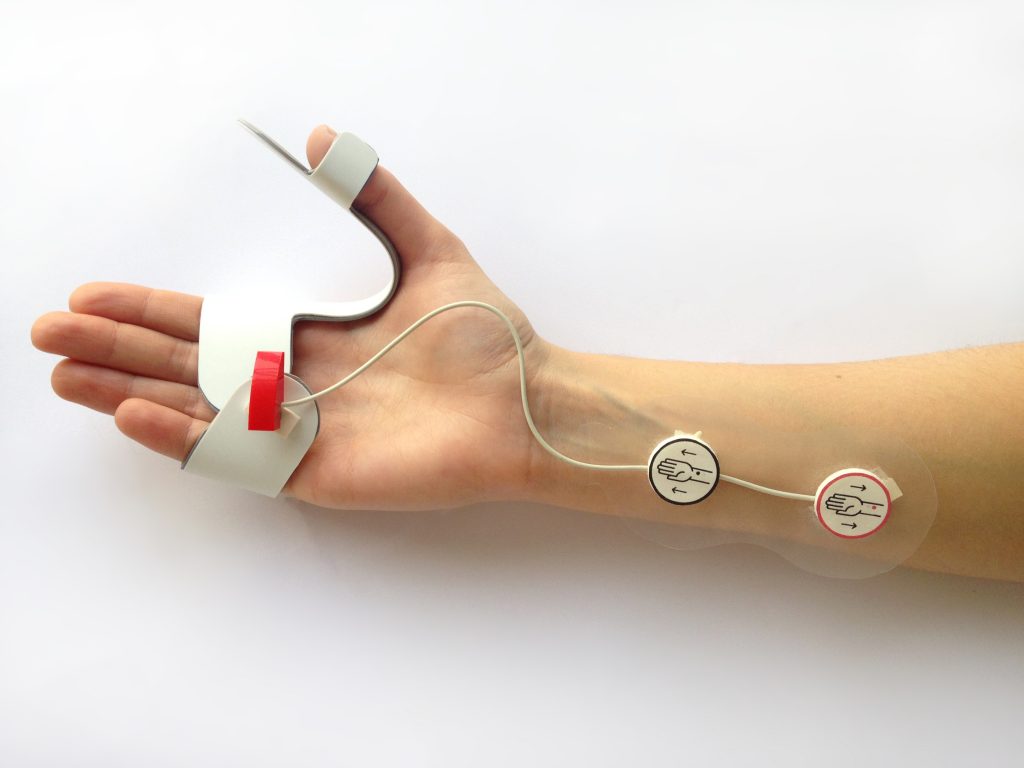
After validating the prototype, we defined the material, characteristics, and connectivity specifications of the three components:
The Stimulator
Adjustable, washable, airtight, shockproof, and bluetooth enabled.
The Electrodes
Butterfly-shaped and disposable to ensure hygiene.
The Interface
General and advanced viewing modes, visual & audible alerts, data export.

We also developed an implementation roadmap, broken up into two phases. The first phase included developing the tablet version of the app to allow an easier adaptation to other devices at a later stage. It would also be an opportunity to develop new features and complementary apps.
01
Stimulator and monitor release
02
Webserver, smartphone and smartwatch app launch
We didn't stop there, however, and suggested three different business models to start developing this solution: Bait & Hook, Freemium, and a Leasing model.
The Leasing and Bait & Hook models would be the most profitable in the short and medium-term, and could bring a faster return on investment. However, since the focus of this product is the democratisation of neuromuscular blockade monitoring, the Freemium model would allow for easier market entry.